R&D (Research and development)
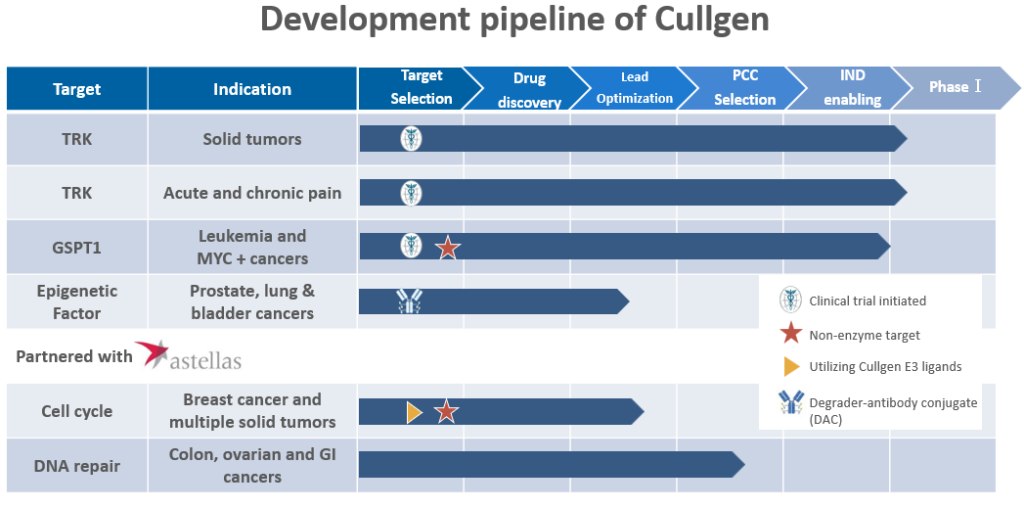
Products/Pipeline
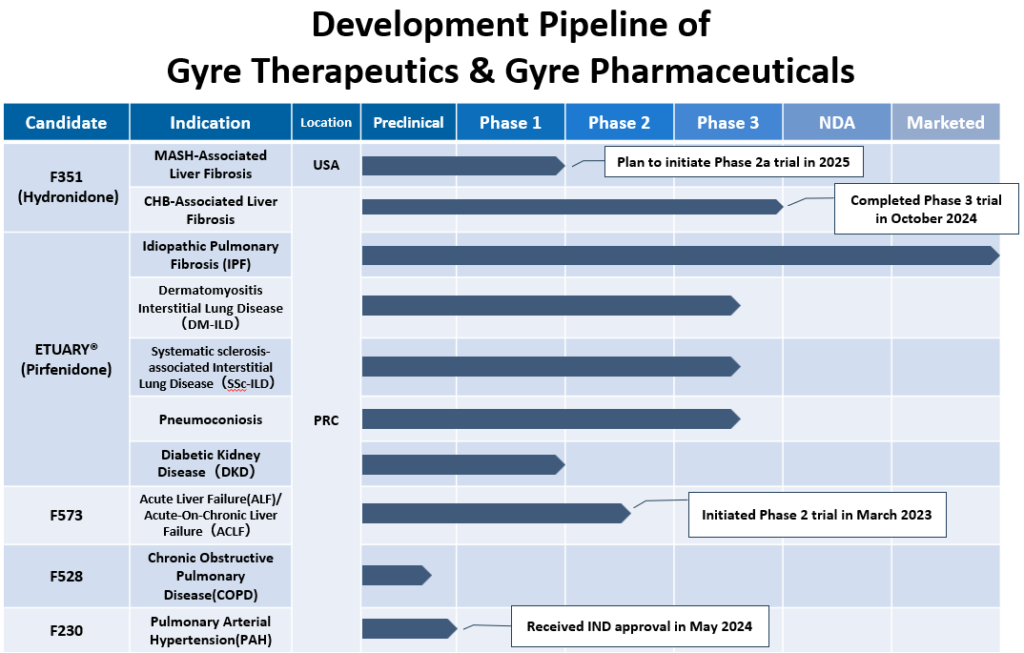
Drug Development Pipeline (As of November 2024)
Products
Approved Drug
- ETUARY®(艾思瑞® in Chinese)
- Indication : Idiopathic Pulmonary Fibrosis (Obtained Manufacturing and Distribution Permission in December, 2013)
(Start selling as the Class 1.1 in February, 2014)
Clinical Development Stage
- ETUARY®(艾思瑞® in Chinese)
- Radiation-induced Pneumonitis:Launched pre-Phase III clinical trial pilot study
IND(Investigational new drug application) Stage
- ETUARY®(艾思瑞® in Chinese)
-
Indication: Diabetic Nephropathy(DN)-Completed PhaseⅠclinical trials
Indication: Connective Tissue Disease Associated Interstitial Lung Disease (CTD-ILD) -PhaseⅢ clinical trials